
- #Negative pressure room guidelines bedside patient care manual
- #Negative pressure room guidelines bedside patient care full
- #Negative pressure room guidelines bedside patient care portable
#Negative pressure room guidelines bedside patient care manual
Manual disinfection alone is unacceptable. EWDs should be used to wash and disinfect all endoscopes following manual cleaning. It is also essential that all channels of all endoscopes are reprocessed after every use of the endoscope, even those that were not used during the preceding patient procedure.ĩ. It is essential that all reprocessing stages are included and documented after every use of the endoscope, and that none is omitted. It is important to ensure that both the endoscope and EWD manufacturers have type- tested the chosen detergents and disinfectants that are compatible for use with their products.Ĩ. Machine testing should include the efficacy and reproducibility of the detergent and disinfectant dosing system, in accordance with the EWD manufacturer’s instructions.ħ. All detergents and disinfectants must be compatible with the EWD and endoscope and used at the correct temperatures and concentrations in accordance with the detergent and disinfectant manufacturers’ instructions. Units should employ single-use disinfectants within purpose-designed washer disinfectors.Ħ. Units should no longer be using aldehyde- and alcohol-based disinfectants because of their fixative properties, which in theory could anchor prion and other proteins within endoscope channels. This routine must be undertaken during lists, between patients and after each patient examination.ĥ. Thorough manual cleaning with a CE marked detergent that is compatible with the disinfectant, including the brushing and flushing of all accessible endoscope channels, must be undertaken before automated endoscope disinfection within an EWD.
#Negative pressure room guidelines bedside patient care portable
Where appropriate quality assurance data are available, the use of CESCs or portable storage systems may obviate the need for repeat endoscope reprocessing at the start of each list.Ĥ.
#Negative pressure room guidelines bedside patient care full
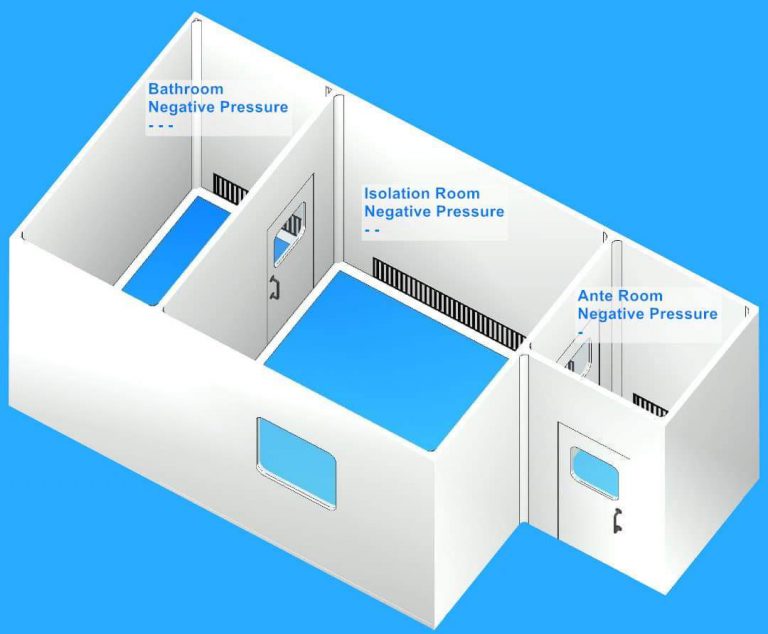
Traditionally, it has been recommended that before the start of each list, each endoscope to be used should undergo a full reprocessing cycle unless last used and decontaminated within the preceding 3 hours. These systems must be checked on a regular basis and validated by the manufacturer.ģ. controlled environment storage cabinets ) or portable storage systems, such as vacuum packing, that may be in use. Training should include an awareness of the channel configuration of all endoscopes, manual cleaning procedures and of the endoscope washer disinfectors (EWD) and available irrigation adaptors, and any post cleaning processes (e.g. Staff training should be implemented using a competency framework and should be documented and revalidated annually. Units should be moving away from single-room facilities and all new designs should have split rooms with clearly segregated clean and dirty areas.Ģ. If a single room procedure is used, the room must be well designed to ensure a good and safe flow is well managed. See Health Technical Memorandum (HTM) 01-06 part B. The washroom area, if separated dirty and clean rooms are used, should have a negative pressure in comparison to the clean side.

Best practice is that there should be physical separation of dirty and clean procedures and areas, each with its own detailed procedures. There should be one- way flow of endoscopes between dirty returns and clean dispatch areas to prevent cross contamination. Decontamination of endoscopes should be undertaken by staff trained and educated in the procedures within dedicated and well-designed rooms. The Report of a Working Party of the British Society of Gastroenterology Endoscopy Committee Summaryġ.


 0 kommentar(er)
0 kommentar(er)
