

Study participants were identified using two management listservs the American Society of Health-Systems Pharmacists and the American College of Clinical Pharmacy. We also assessed the adherence to the 2009 Expert Consensus Guidelines for Stocking Antidotes in Hospitals that Provide Emergency Care.Ī web-based electronic survey was sent out to hospital pharmacy directors/managers at hospitals with an emergency department. Specifically, we sought to identify the influence of catchment area, trauma center designation, hospital size, clinical pharmacist and subspecialist employment, funding source, and other hospital characteristics on stocking choice and level. The aim of our study was to survey pharmacies from US hospitals that provide emergency care, to determine which factors or barriers influence cyanide antidote stocking decisions, and to identify stocking patterns. Hydroxocobalamin was designated as the preferred agent because of its wider application (smoke exposure), ease of use, and anticipated safety in widespread use, though both the CAK and hydroxocobalamin were recognized as acceptable. Factors to consider when assessing a hospital's need to stock antidotes include characteristics of the catchment area (industries, indigenous fauna, etc.,), history or experience of using the antidote, and anticipated time to restock the antidote. Thus, the experts recommend that each hospital perform a hazard vulnerability assessment (HVA) to determine the amount of antidote to be stocked. A rigid stocking recommendation for all hospitals may lead to insufficient stocking. In addition to cyanide antidotes, the diverse panel of experts considered 24 antidotes for stocking and recommend that 12 be available for immediate administration. Thus far, no published study has evaluated compliance of hospital cyanide antidote stocking levels with the 2009 expert consensus guidelines which recommends using two kits of hydroxocobalamin or one CAK to treat a 100 kg patient. To date, no analysis has specifically analyzed cyanide antidote stocking, but several surveys have concluded that, in general, antidote stocking throughout North America is insufficient. A recent animal study suggests that co-administration of hydroxocobalamin and sodium thiosulfate has similar early clinical improvement as sodium thiosulfate and sodium nitrite. Comparative data supporting one preferred antidote are low quality and limited. Unfortunately, it is difficult to determine if hospitals stock sufficient quantities of an “appropriate” cyanide antidote because debate exists over which is more effective and safe. Hydroxocobalamin binds directly to cyanide to form cyanocobalamin, which is non-toxic and excreted in the urine.īecause cyanide is ubiquitous and is a frequent co-intoxication in victims of smoke inhalation, adequate hospital stocking of an effective antidote for cyanide intoxication is essential. Sodium thiosulfate replaces depleted sulfate groups necessary for conversion of cyanide to thiocyanate, a less toxic and renally excreted compound.
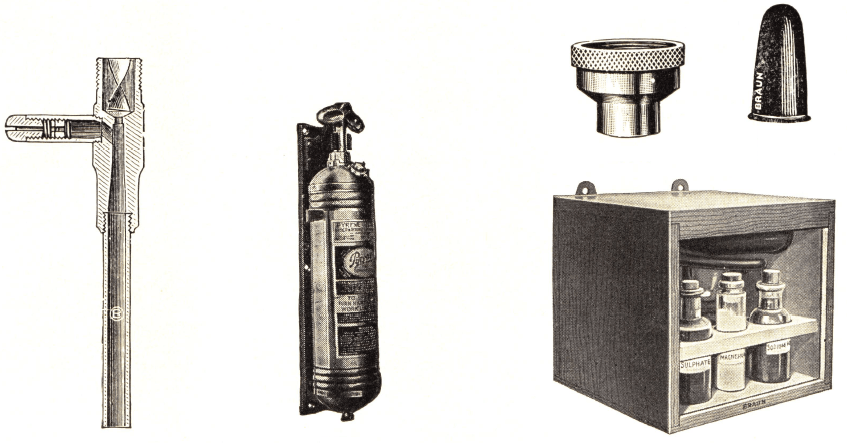
Their respective prices are approximately $20, $110, and $750. The three antidotes currently used in the United States are sodium thiosulfate (25% solution for injection), The cyanide antidote kit (CAK) (sodium nitrite and sodium thiosulfate), and hydroxocobalamin (CYANOKIT ®). Because diagnosis is difficult, an antidote that is both effective and safe is desirable for empiric treatment. Cyanide causes a shift towards cellular anaerobic metabolism, lactic acidosis, and rapid and severe central nervous system, cardiovascular, and respiratory toxic effects.

In 2009, 238 exposures were reported to US poison control centers (PCC) and 70% of these were unintentional though it has the potential to be used as a chemical weapon. It has been used as an agent for suicide, homicide, and terrorism. It contributes to smoke inhalation injury as the burning of plastics, silk, wool, and cotton releases hydrogen cyanide into fire smoke. It is used in mining, pest control, and industry. Cyanide is a potent toxin that occurs in numerous forms causing harm if exposed by ingestion, inhalation, dermal absorption, or parenteral administration.


 0 kommentar(er)
0 kommentar(er)
